Opening comments
LNG stands for Liquefied Natural Gas. It is natural gas, primarily methane, that has been cooled to a liquid state at about -162°C for shipping and storage. The volume of natural gas in its liquid state is about 600 times smaller than its volume in its gaseous state, making it easier for road, rail, or ocean transport. LNG is a clear, colourless, and non-toxic liquid that will not ignite in its liquid state. When LNG reaches its destination, it is sold as LNG fuel, or turned back into a gas at regasification plants.
LNG’s extremely low temperature makes it a cryogenic liquid. Generally, substances which are -100°C or less are considered cryogenic and involve special materials and technologies for handling.
Density and specific gravity
Density is a measurement of mass per unit of volume and is an absolute quantity. Because LNG is not a pure substance, the density of LNG varies slightly with its actual composition. The density of LNG falls between 430 kg/m3 and 470 kg/m3. LNG is less than half the density of water; therefore, as a liquid, LNG will float if spilled on water.
Specific gravity is a relative quantity. The specific gravity of a liquid is the ratio of density of that liquid to density of water at 15,6°C. The specific gravity of a gas is the ratio of the density of that gas to the density of air (at 15.6°C). Any gas with a specific gravity of less than 1,0 is lighter than air (buoyant). When specific gravity or relative density is significantly less than air, a gas will easily disperse in open or well-ventilated areas. On the other hand, any gas with a specific gravity of greater than 1,0 is heavier than air (negatively buoyant). The specific gravity of methane at ambient temperature is 0,554, therefore it is lighter than air and buoyant.
LNG vapours at the boiling point temperature (-162°C) and atmospheric pressure have a relative density of about 1,8, which means that when initially released, the LNG vapours are heavier than air and will remain near the ground. However, as methane vapours begin to rapidly warm and reach temperatures of approximately -110°C, the relative density of the natural gas will become less than 1 and the vapours become buoyant.
At ambient temperatures, natural gas has a specific gravity of about 0,6, which means that natural gas vapours are much lighter than air and will rise quickly. Cold LNG vapours (below -110°C) are negatively buoyant and more likely to accumulate in low areas until the vapours warm. Therefore, a release of LNG that occurs in an enclosed space or low spot will tend to replace the air (and oxygen) and make the area a hazard for breathing.
Flammability
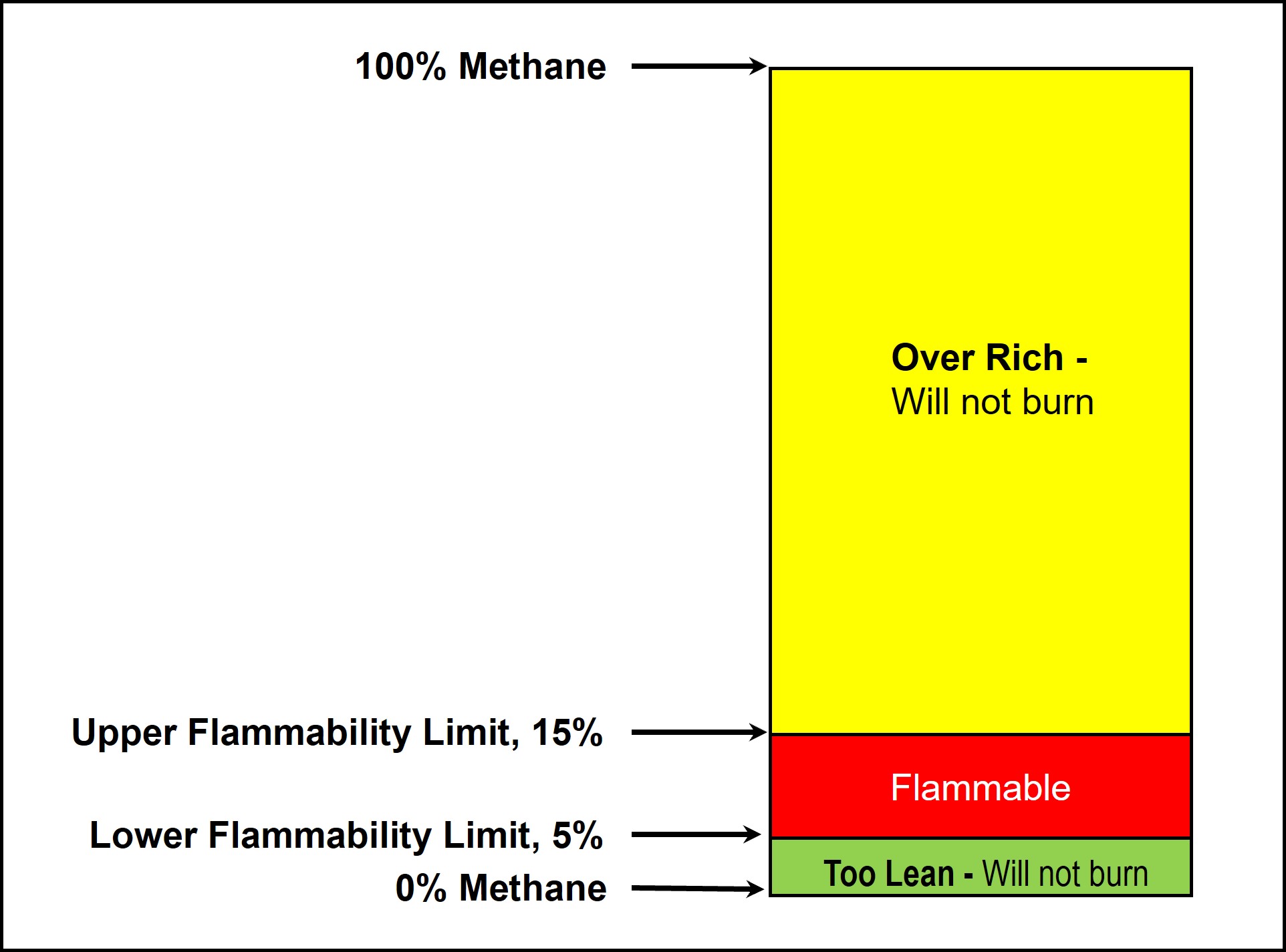
Flammability is the property which makes natural gas desirable as an energy source, and yet for the same reason flammability can be a safety hazard. Note that natural gas is flammable, but LNG (the liquid form of natural gas) is not because of the lack of oxygen in the liquid. Since LNG begins vaporising immediately upon its release from a container, the important issue is when will the vapours be flammable and for how long?
Several factors are required to start a fire from LNG vapours, namely fuel (LNG vapours), oxygen and an ignition source. The fuel and the oxygen have to be in a specific range to form a flammable mixture. This flammable range is the range of a concentration of a gas or vapour that will burn if an ignition source is introduced. The limits are commonly called the “Lower Flammable Limit” (LFL) and the “Upper Flammable Limit” (UFL). The flammability limits for methane are 5% LFL and 15% UFL by volume in air, as shown in the diagram above. Outside of this range, the methane/air mixture is not flammable.
Auto-Ignition temperature
The auto-ignition temperature is the lowest temperature at which a gas or vapour in air (e.g., natural gas) will ignite spontaneously without a spark or flame being present. This temperature depends on factors such as air-fuel mixture and pressure. In an air-fuel mixture of about 10% methane in air, the auto ignition temperature is approximately 540°C. Diesel oil and gasoline will auto-ignite at substantially lower temperatures than LNG.
In addition to ignition from exposure to heat, the vapours from LNG can be ignited immediately from the energy in a spark, open flame, or static electricity when they are within the flammable limits.
LNG safety
The LNG industry has an excellent safety record over the past 50 years. However, LNG has some safety hazards that need to be considered, such as:
- Fire and explosion: LNG itself does not burn, but the boil-off natural gas does. Natural gas can ignite if it encounters an ignition source, such as a spark or flame. LNG can also form a flammable vapour cloud that can travel and explode if ignited.
- Cryogenic freeze burns: LNG is very cold (-162°C) and can cause severe frostbite or tissue damage if it touches the skin or eyes.
- Embrittlement of metals and plastics: LNG can make some materials brittle and prone to cracking or breaking if they are not designed to handle low temperatures.
- Asphyxiation: LNG vapours or boil-off natural gas can displace oxygen in the air and create a risk of suffocation for people or animals in the vicinity.
- Rapid phase transition: LNG can rapidly regasify and create a shock wave if it meets water or warmer liquids.
These hazards can be mitigated by following proper safety procedures, such as using appropriate materials, equipment, and personal protective equipment, avoiding ignition sources, monitoring gas concentrations, and maintaining safe distances.
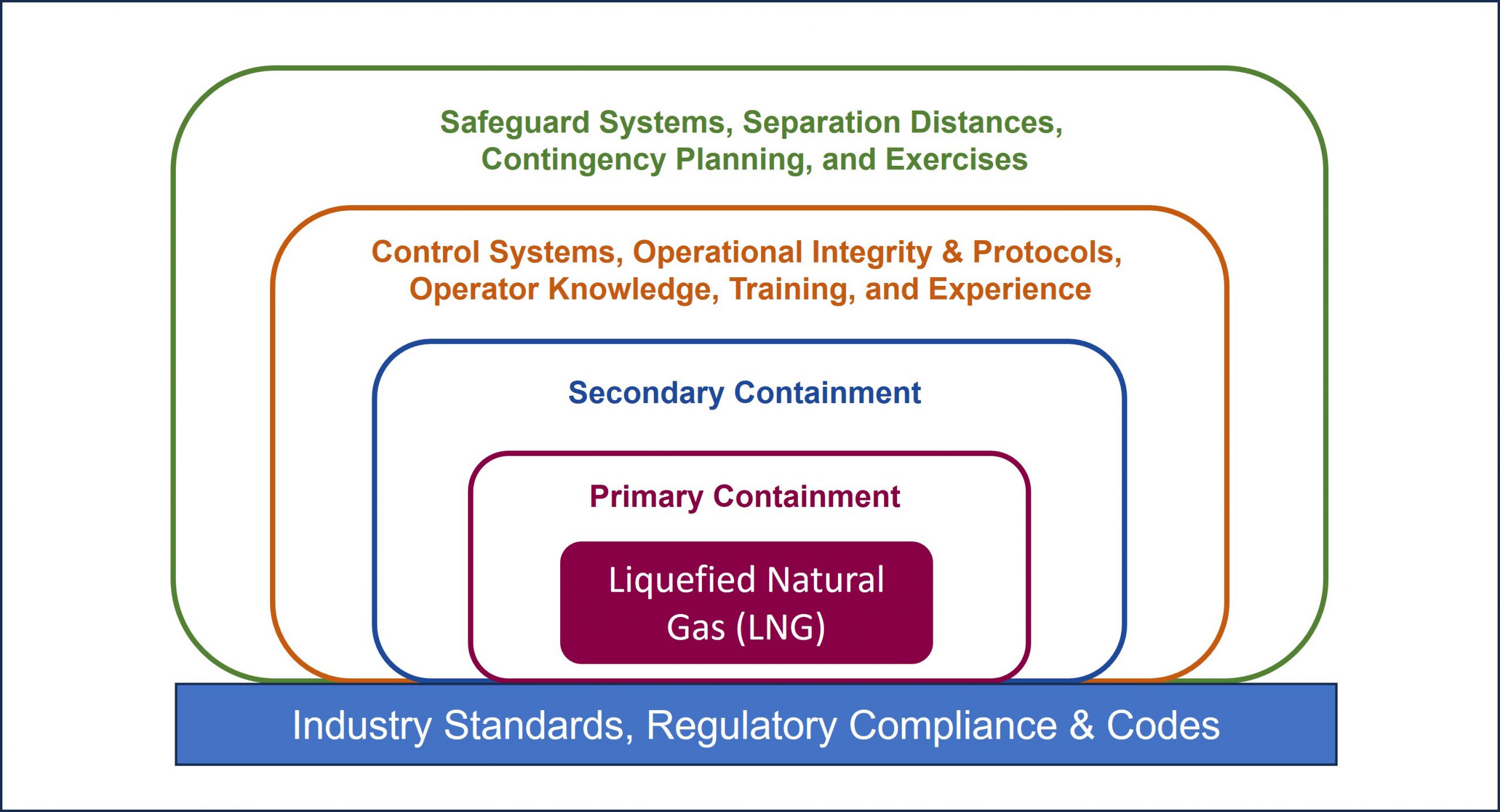
The safety record of LNG worldwide is a result of proven and detailed industry standards, strong regulations, and an industry commitment to risk management. For all LNG facilities, multiple layers of protection are implemented to minimize the likelihood of an LNG release and, if a release occurs, to mitigate the consequences. The multiple layers of protection are illustrated in the diagram below.
Conversion factors
Some useful conversion rates for LNG are shown in the table below.
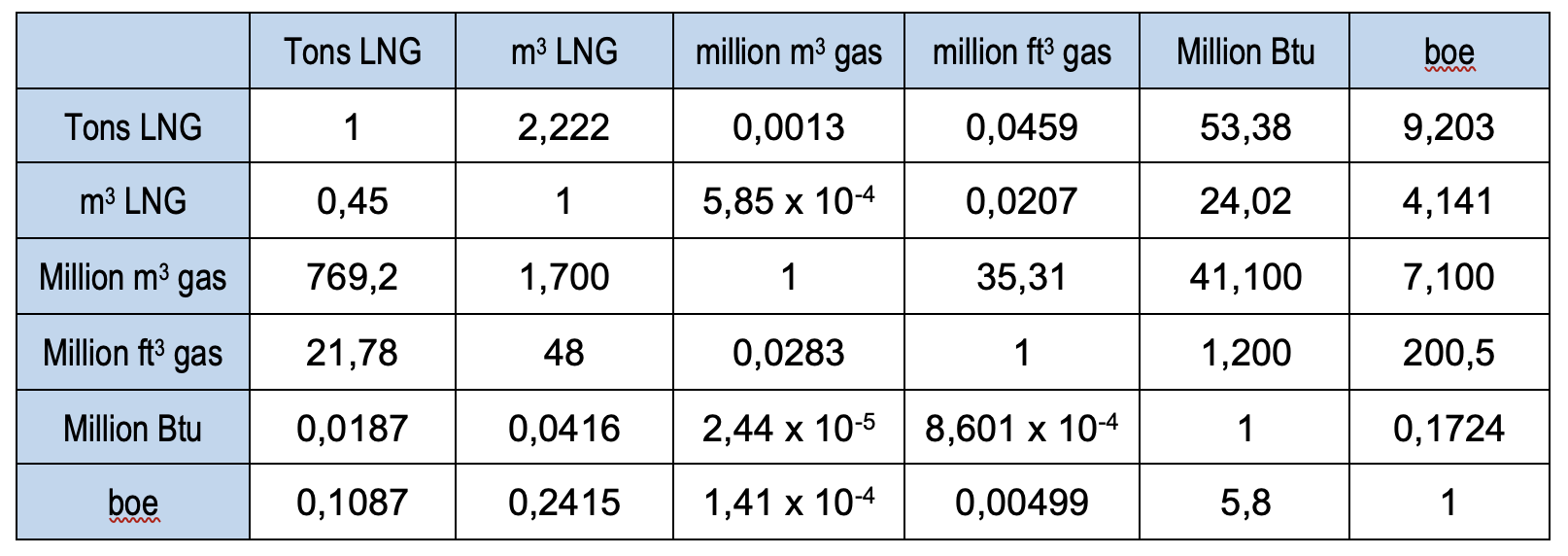
Btu = British thermal units
boe = barrels oil equivalent
info@polairetech.com

